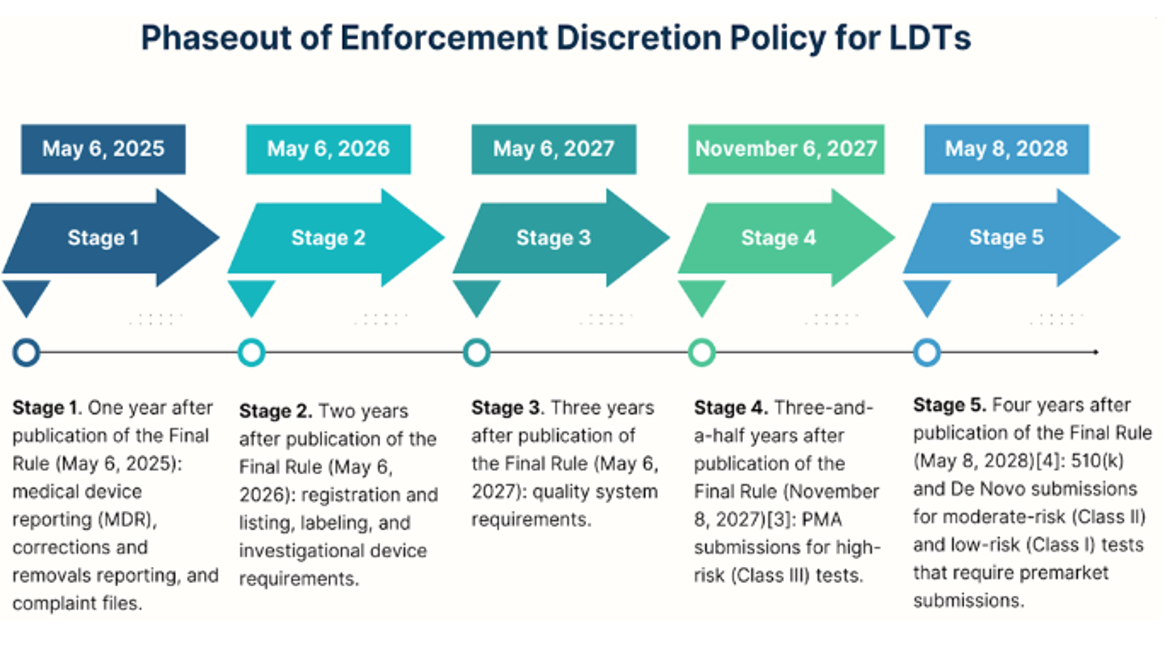
In our previous blog in this series, we summarized the FDA’s plan to phase out the enforcement discretion policy for LDTs. To recap, the phase-out policy involves five stages spread across five years.
In this blog, we will elaborate on which rules apply to the various types of LDTs.
The types of compliance required are divided into Tiers 0-IV, as shown in the table below.
Tier 0 lists tests for which the compliance required by the final rule is already expected and enforced.
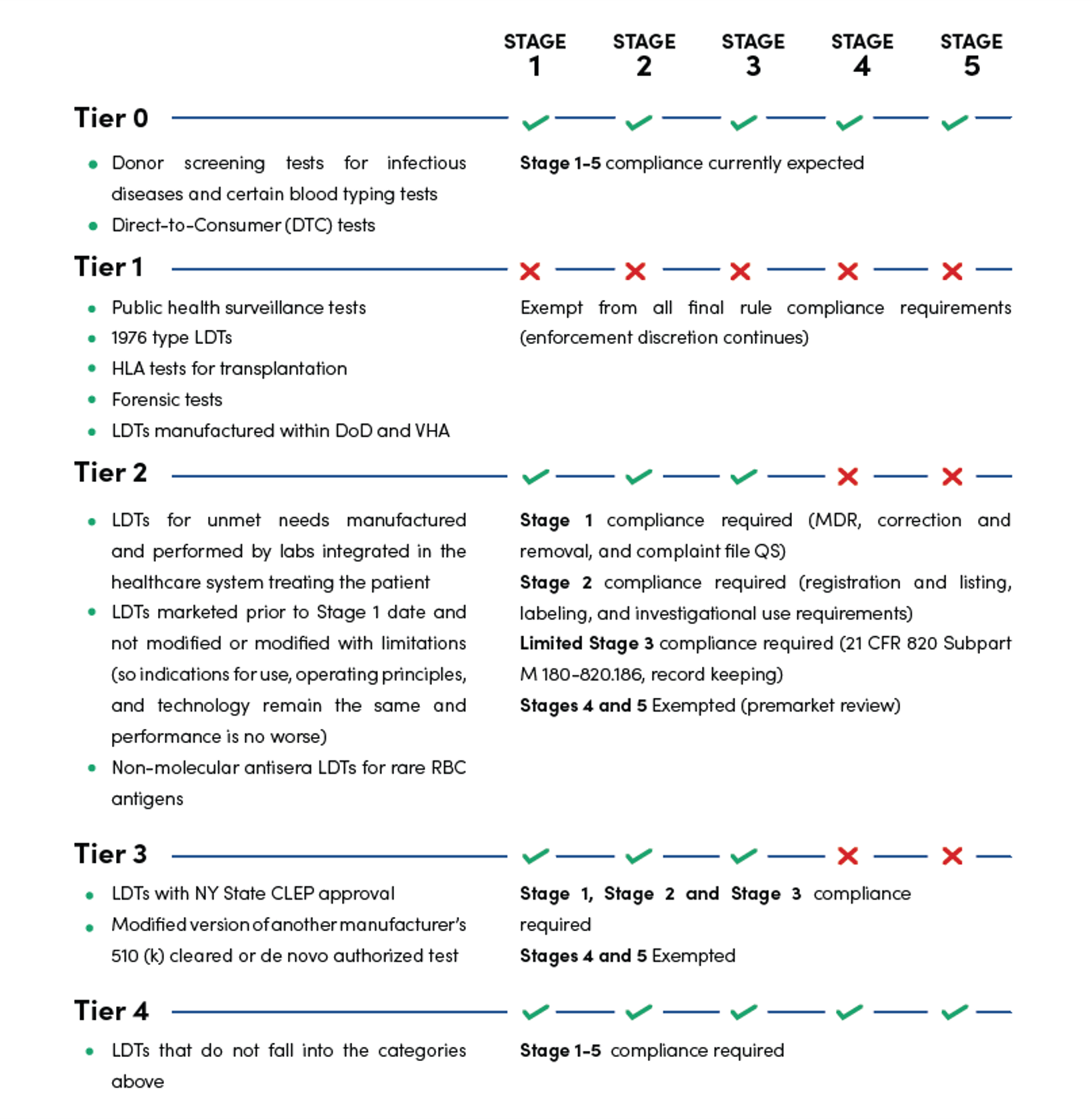
Thus, the stringency of compliance depends on the type of LDTs being manufactured.
If you are a new molecular LDT manufacturer, watch out for our next blog, which will elaborate on the specific processes that need to be set up and, importantly, how Strand can help.
Explore the rest of the blog series below: