Quick, Actionable qPCR Reporting At Your Fingertips
Quick, Actionable qPCR Reporting At Your Fingertips
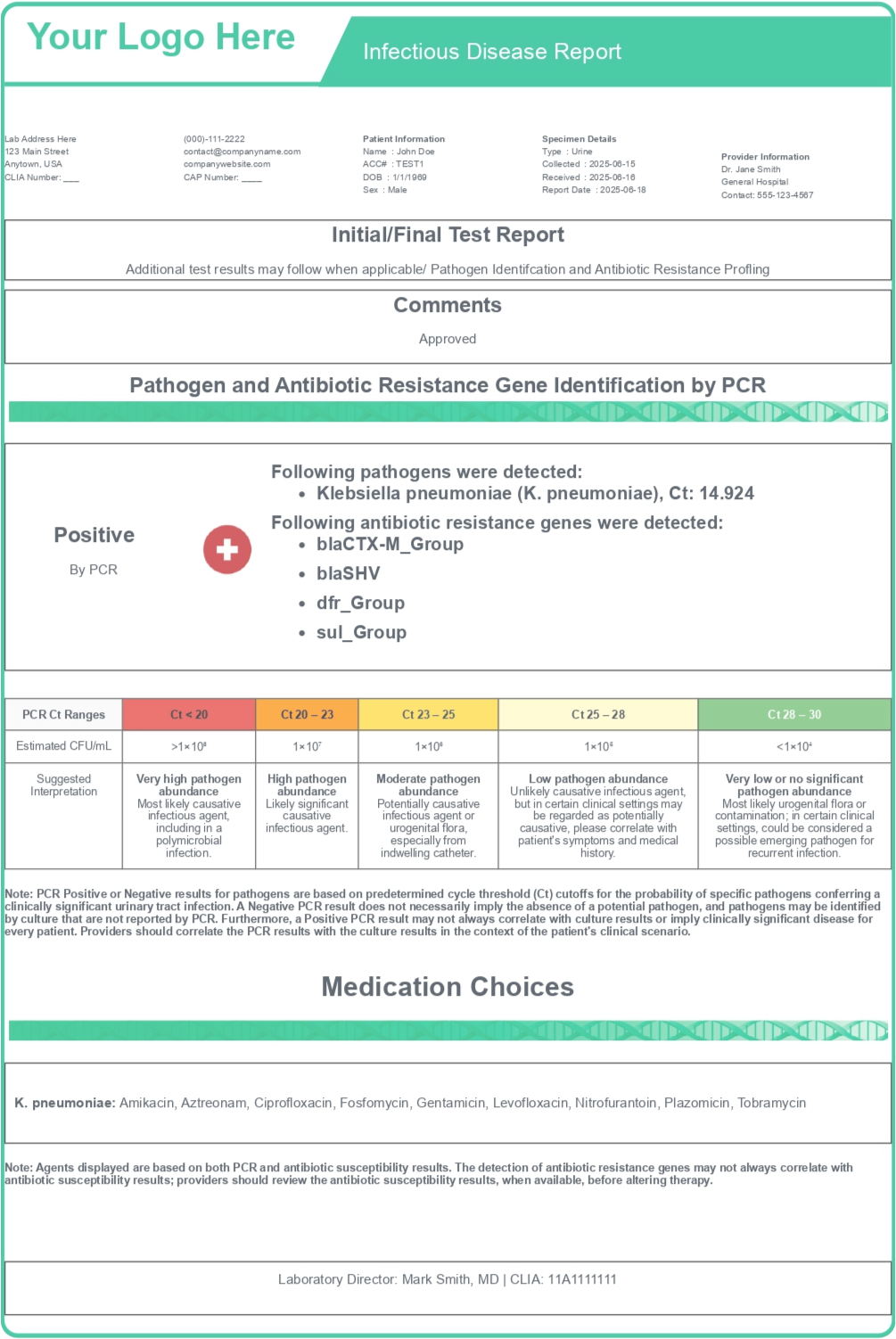
Fast Facts
Fast Facts

Scalable to Handle Additional Tests and Increasing Volumes

150-200
Able to generate hundreds of reports a month, with the ability to scale as needed.

Extensive
Customization

Quick and
Easy
Set Up
Available in as Little as a Week

Easily Integrated Into LIMS Using a RESTful API

Continual Client Support, Including New Feature Requests and Troubleshooting

2 Mins
Report TAT

Supports a Range of Tests
Workflow
Workflow
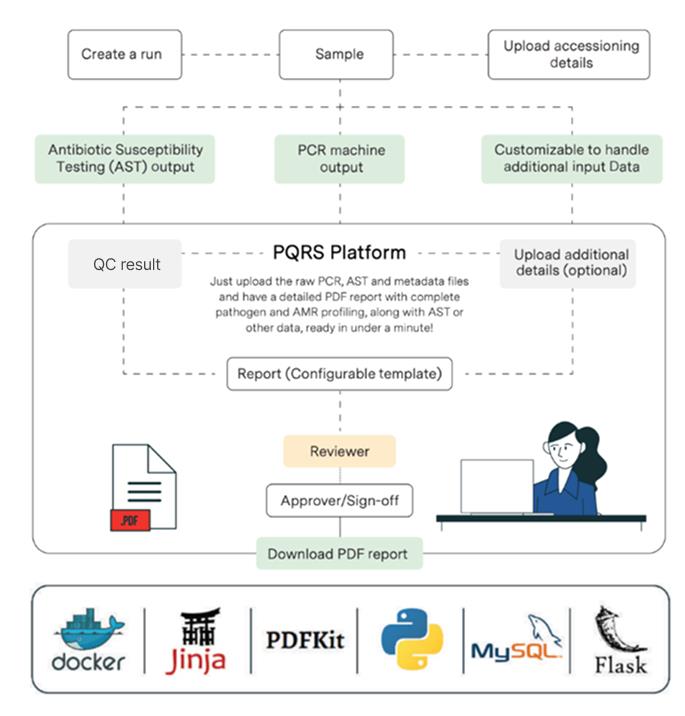
The process is initiated by inputting qPCR or antibiotic susceptibility testing (AST) output files. If required, the PQRS platform can be customized to handle any other kind of input data as well.
Manual QC is carried out based on the positive and negative controls available in the input file.
A report is then compiled and generated based on a configurable template.
Finally, the report is made available to the end user as a PDF, ready for a quick review process by a senior scientist.
All of this is done within 2 minutes
Reports
Reports
Hover on each info to view more information
Three Key Benefits of our Fully Customizable Reports:
Three Key Benefits of our Fully Customizable Reports:

01
Clinician-approved format
We incorporate client-specific requirements into the qPCR report through frequent interactions with the clinician team.

02
Combined qPCR and AST reporting
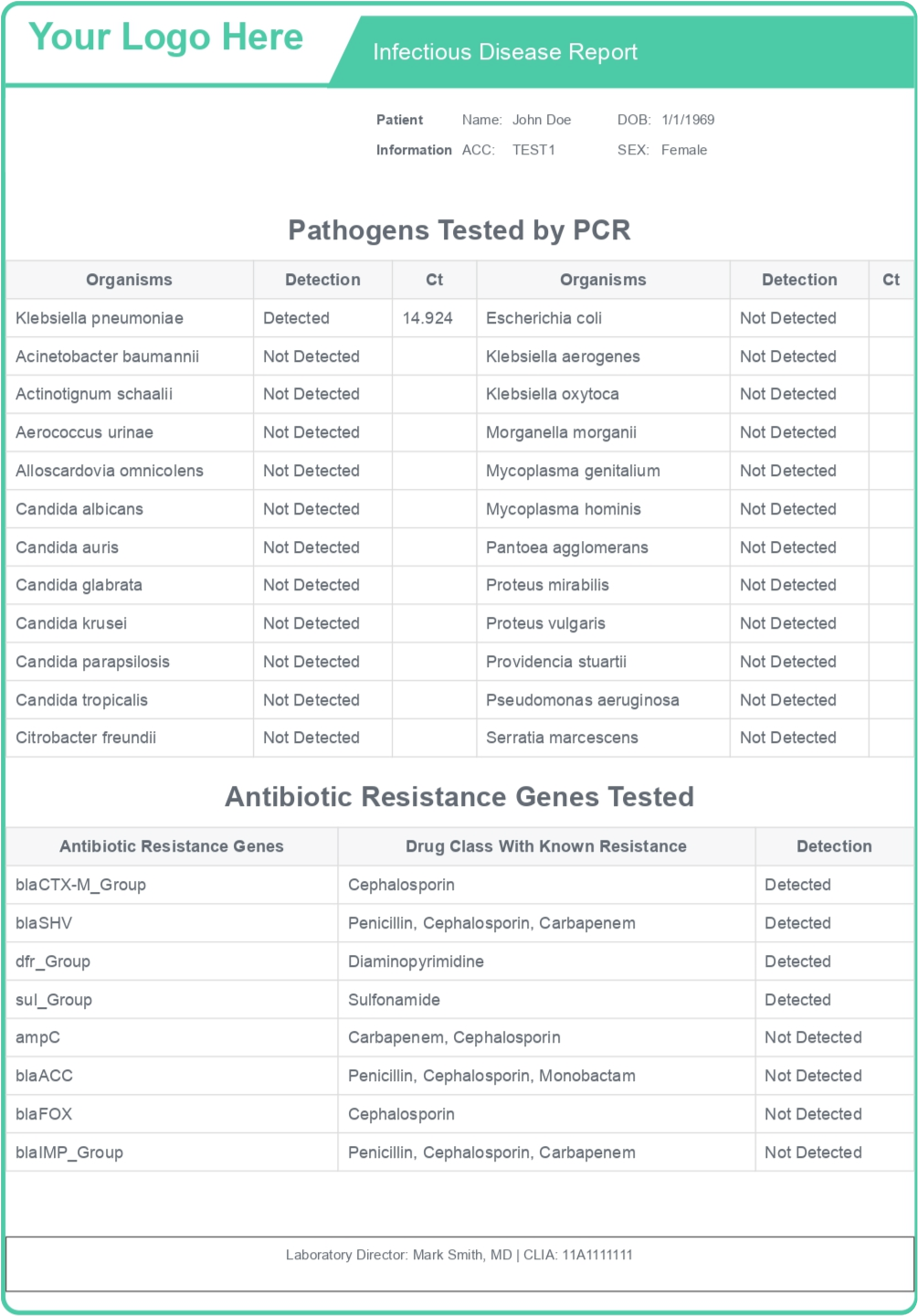
The end report captures the qPCR-identified pathogens, antibiotic resistance genes, as well as antibiotic susceptibility test results, offering insights on infectious pathogens as well as AST status.

03
Broad array of medication options
Clinicians can choose from multiple antibiotic recommendations, enabling them to freely make patient-tailored decisions.
Solutions Tailored to Client Needs

Developing a template that works for our client, such as adding:
- Client Logo
- Laboratory Signature
- Disclaimers
- Methods Section

Modifying our curated reference knowledge base, catering specifically to the client’s panel manifest and updated treatment guidelines.

Including reporting on the antibiotic susceptibility test. In essence, if the input file contains the antibiotic susceptibility profile for specific pathogens, our PQRS solution, empowered by the curated KB, generates reports with optimized medication choices.

Enabling panel-specific updates to PQRS, i.e., ensuring that all the pathogens/ AMR genes in the client’s panel of interest are covered in the reporting solution and can be regularly updated

Addition of audit logs to easily track the time when reports are created and sign-offs are completed.

Incorporate a provision for Subject Matter Expert sign-off in addition to the standard approvals

Offering continued support to the client’s team and promptly addressing any issues and additional feature requests.
Features
Features
Reference Databases
- The PQRS platform is equipped with a curated infectious-disease knowledge base database that serves as the foundation for compiling reports.
- These KBs can also be customized as per client preferences.
- Strand’s curation scientists and reviewers built this foundational database by gathering information from several well-known publicly available reference databases for infectious disease reporting, including:
Compliances
- Our privacy and security protocols ensure patient data remains confidential as per HIPAA guidelines.
- The data received through our API is also HL7 compliant.
Additional Input and Output Options
- Depending on the data you would like to analyse we can take in additional files, such as antibiotic susceptibility testing files
- Additional results can be included such as demographic breakdowns and dosage recommendations
Reference Databases
- The PQRS platform is equipped with a curated infectious-disease knowledge base database that serves as the foundation for compiling reports.
- These KBs can also be customized as per client preferences.
- Strand’s curation scientists and reviewers built this foundational database by gathering information from several well-known publicly available reference databases for infectious disease reporting, including:
Compliances
- Our privacy and security protocols ensure patient data remains confidential as per HIPAA guidelines.
- The data received through our API is also HL7 compliant.
Additional Input and Output Options
- Depending on the data you would like to analyse we can take in additional files, such as antibiotic susceptibility testing files
- Additional results can be included such as demographic breakdowns and dosage recommendations
Frequently Asked Questions
Frequently Asked Questions
FAQ Categories
No FAQs available for this category.