Cancer—a small word with devastating consequences. In 2020 alone, 18.21 million people were diagnosed with cancer, while nearly 10 million lost their lives [1,2]. Cancer is the second leading cause of death globally. Survival rates depend on multiple factors including the stage and site of the cancer, patient age, health, and family history, among others. One in every nine Indians faces the risk of developing cancer in their lifetime, with breast, lung, and colon cancers being the most prevalent [3].
Clinically, cancer is still classified according to the tissue or organ of origin. Yet, our strategy for understanding and tackling cancer is on the brink of a transformation. At the core of this change is a well-established piece of knowledge: cancer is fundamentally a disorder of the genetic machinery of cells [4].
Cancers are caused by uncontrolled cell proliferation that occurs when ‘driver’ mutations constitutionally activate oncogenes, or when ‘suppressor’ mutations inactivate tumor suppressor genes. These mutations may take the form of single nucleotide variants (SNVs), insertions, deletions, gene rearrangements, or copy number variations (CNVs).
In 5-10% of cases, these mutations are inherited; however, most are somatic mutations that arise de novo in response to internal or external stressors, or due to erroneous DNA mismatch repair [5]. Mutations that enable cells to evade replication checkpoints or offer survival benefits are crucial for tumor progression to cancer.
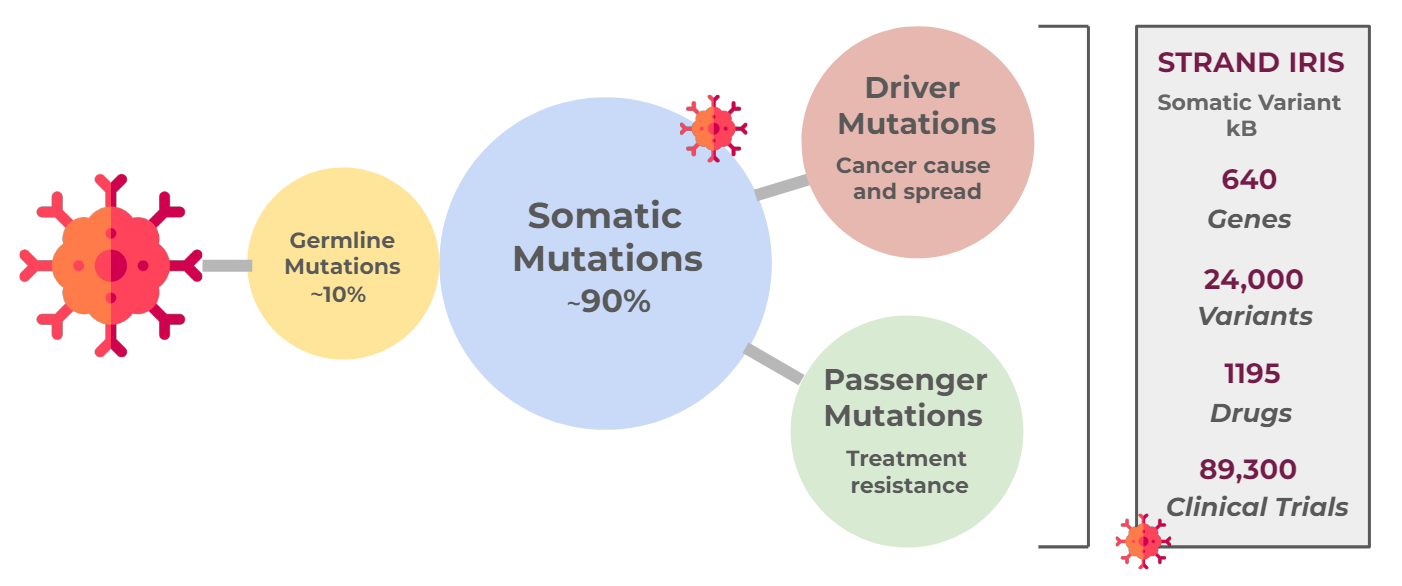
Even for an individual patient, cancer is a highly heterogeneous condition. Cells from different tissues may have similar underlying cancer mutations. Conversely, as tumors progress, various mutations can accumulate because cells replicate faster than error repair systems can correct. Mutations may vary between primary and metastatic tumors, among metastatic tumors in different tissues, and even among cells within the same tumor, leading to treatment resistance. The exact combination of mutations is unique to every patient.
Thus, each patient harbors a distinct profile of mutations that reflects the origin, characteristics, progression, spread, and pathogenicity of their cancer—a kind of molecular fingerprint that can be decoded from the genomes of tumor cells or circulating cell-free tumor DNA.
Identifying this genetic signature enables us to target the core mechanisms driving the cancer with specific, timely treatments. This approach, made possible by Next-Generation Sequencing (NGS), is known as precision oncology. Ideally phrased, “the goal of precision medicine is simply to deliver the right cancer treatment to the right patient at the right dose and at the right time.” [4]
Precision oncology assesses how 'actionable' cancer mutations are, i.e., whether they can influence treatment decisions. Examples include established variants in genes such as EGFR, ALK, MET, ROS1, NTRK, HER2, KIT, BRAF, and BRCA1/BRCA2. These variants help determine the choice between cytotoxic, immunologic, or biologic treatment options for various cancer subtypes. Every year, new variants like the KRAS A146T, implicated in colorectal adenomas, are added to this arsenal [5].
Effective implementation of precision medicine begins with genetic testing of patients, followed by the interpretation of results to identify appropriate, targeted treatments, and culminates in the application of these treatments. Understanding the interpretation process is crucial, as it involves scanning thousands of somatic variants across multiple databases to accurately identify potential matches. Strand's Iris platform offers a fully automated system that can analyze, interpret, and report actionable oncological variants from NGS tests with remarkable accuracy and rapid turnaround.
The best cancer prognosis hinges on precise information and prompt action—detecting risks early, diagnosing, monitoring progression, targeting treatments, evaluating efficacy, adjusting doses, and predicting treatment resistance. Precision oncology has the potential to revolutionize each of these aspects, and we at Strand are here for it!
You can find more details regarding StrandIris, here!
Explore the rest of the blog series below:
- Part 2: Somatic Variants and Databases: A Wealth of Information
- Part: 3: Somatic Variant Classification—Mining for Clues
- Part 4: Somatic Variants: Towards Better Therapy
References:
- WHO Cancer: Key facts
- World Cancer Research Fund International- Worldwide cancer data
- Sathishkumar, K., Chaturvedi, M., Das, P., Stephen, S., & Mathur, P. (2023). Cancer incidence estimates for 2022 & projection for 2025: result from National Cancer Registry Programme, India. Indian Journal of Medical Research.
- Schwartzberg, L., Kim, E. S., Liu, D., & Schrag, D. (2017). Precision oncology: who, how, what, when, and when not? American Society of Clinical Oncology Educational Book, 37, 160-169
- Kalmár, A., Galamb, O., Szabó, G., Pipek, O., Medgyes-Horváth, A., Barták, B. K., ... & Takács, I. (2023). Patterns of Somatic Variants in Colorectal Adenoma and Carcinoma Tissue and Matched Plasma Samples from the Hungarian Oncogenome Program. Cancers, 15(3), 907.












