Qualitative PCR or qPCR is a widely used molecular diagnostic method for rapid and high-throughput detection of target DNA, across applications like gene expression analysis, pathogen detection & surveillance, strain typing and antimicrobial resistance profiling. qPCR plays a key role in enabling faster results especially in infectious disease testing where quick and accurate identification of pathogens is critical.
However, despite its clinical value, qPCR workflows still rely heavily on manual interpretation and reporting, which is time-consuming and demands significant expertise. Automation in these steps is still limited, leading to delays in reporting and increased workload for lab scientists. Additionally, supporting a wide range of labs, each with their own curated databases of pathogens and antibiotics, through a single portal adds to the challenge.
Modular systems that enable automated interpretation and reporting, while significantly reducing turnaround times, are, therefore, the need of the hour.
At Strand, we’ve developed PQRS, a qPCR reporting platform that allows multiple labs to use the same application for automated interpretation and reporting. Reducing manual effort and delivering turnaround times of ~2 min from input to report generation, its modular design also makes it easy to onboard labs with their own curated pathogen and antibiotic databases across disease panels, offering the flexibility to support different reporting needs. Its key features include:
1. Flexible Input Support: Accepts a wide range of input file formats, including CSV and TSV, with the option to add more based on customer requirements.
2. LIMS Integration: Offers APIs (RESTful API) for easy LIMS or internal system integration, enabling direct report generation from sample data.
3. Scalability: Efficiently handles additional tests and high volumes of report generation.
4. Extensive Customization: Offers amenability, by allowing us to tailor report templates and workflows to each lab’s needs, onboarding labs with minimal customizations in just 2 weeks and more complex ones under 3 months, along with continued support post-onboarding.
Some of the customizations that can be made include:
- Developing a template that is suited to the needs of the client
- Modifying our curated reference knowledge base, to align with the client’s panel and treatment guidelines
- Including antibiotic susceptibility data to generate optimized medication recommendations
- Dosage recommendations
- Enabling panel-specific updates to PQRS, to ensure all pathogens/ AMR genes in the client’s panel of interest are covered
- Adding a Subject Matter Expert (SME) sign-off step
- Incorporating audit logs to track report creation and approvals
- Providing ongoing support for issue resolution and new feature requests
5. Curated Infectious Disease Knowledgebase for Reliable Reporting: Comes equipped with an infectious disease knowledgebase curated by Strand’s scientists and reviewers, using trusted, publicly available databases like Sanford Guide, Medscape eMedicine and Johns Hopkins Guides to ensure reliable reporting.
6. HIPAA-Compliant Data Privacy: Complies with HIPAA guidelines by ensuring patient data always remains confidential, through our privacy and security protocols.
The PQRS Workflow:
The fully automated workflow initiates with the ingestion of qPCR output files; Antibiotic Susceptibility Testing (AST) data, raw result files, metadata, or any other input types as per the customization requirements. Once the data is uploaded, QC is carried out based on the positive and negative controls available in the input file to ensure accuracy. A report is then compiled using a configurable template, quickly reviewed by a Senior Scientist and made available to the end user as a PDF. A built-in role-based access control system allows authorized users of the lab, such as reviewers and approvers, to then validate and approve the report before it’s finalized, to ensure accuracy.
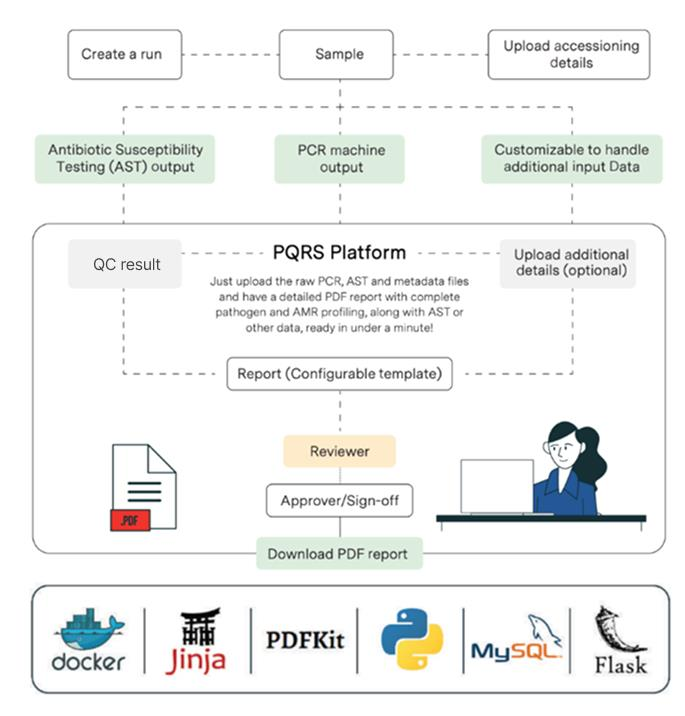
The PQRS Report is organized into three main sections. The first covers patient details, specimen information, and provider data. The second lists pathogens and antibiotic resistance (AMR) genes detected through PCR. And, the third section outlines the medication options optimized to treat the pathogen. The first page presents a high-level summary, with subsequent pages providing more detailed pathogen and resistance information.
Our customizable qPCR report offers three key advantages:
- Clinician-approved format:
We’re constantly in touch with the associated clinical team to incorporate client-specific requirements into the qPCR report. - Integrated qPCR and AST reporting:
The report includes both pathogen and resistance gene data from qPCR, along with AST results, providing insights on the pathogens as well as the treatment options based on the AST status - Broad range of medication options:
Clinicians get a range of antibiotic recommendations, allowing for greater flexibility in choosing the most appropriate treatment for each patient.
Together, these features make qPCR reporting not only faster, but more actionable, helping clinicians move quickly from diagnosis to treatment, and offering a practical solution to the challenges faced in qPCR interpretation and reporting.
Download a PDF version of our PQRS brochure from our website or connect with us to explore how we can help you accelerate qPCR interpretation and reporting!