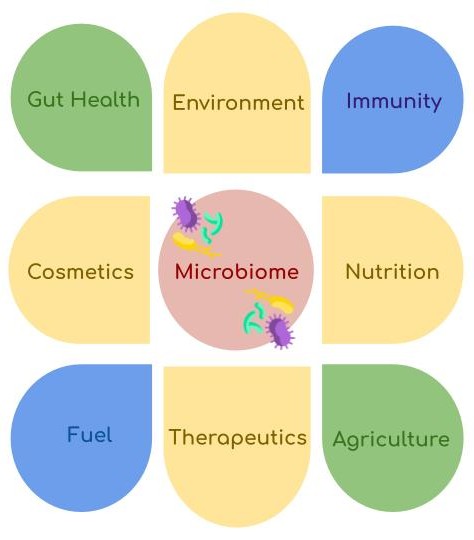
Microbiota refers to the community of microorganisms, including bacteria, archaea, protozoa, fungi, and algae, found in a specific area in the body or in an ecosystem. In humans, this primarily includes bacteria and archaea residing in places like the mouth, gut, or skin. The term "microbiome" encompasses this entire ecological system, which includes microorganisms and their unique habitats, along with various structural components and metabolites. The complex interaction between human cells and commensal microorganisms is referred to as a ‘holobiont’, and the human microbiome is even called the "last organ" because of its absolutely vital role in health. Overall, the microbiome plays a crucial role in human health, energy production, agriculture, and environmental stability.
In the 20th century, culture techniques with limited resolution and throughput were the mainstay for identifying microbiota. NGS, along with advances in metabolomics, transcriptomics, and proteomics, have revolutionized microbiome research by enabling cost-effective, reliable, high-throughput analysis. This progress has paved the way for metagenomics, the comprehensive examination of all genetic material from environmental or biological samples. We can now perform detailed characterization of microbial communities and their functional capacities across various spheres.
The microbiota is integral to the biology, development, physiology, and disease processes of its human host. Imbalances in the microbiome have been associated with a plethora of conditions such as cancer, heart disease, inflammatory bowel disease, liver disease, chronic kidney disease, brain disorders, diabetes, and respiratory disease. Over 15% of the global cancer burden is estimated to be linked to microbes. Metagenome analysis can identify and analyze microbial diversity in cancer tissue, potentially establishing connections between specific microorganisms and different cancer types, which could enhance drug target development and selection.
The commensal microbiome can influence the effectiveness of immunotherapy by affecting immune checkpoint disruptions critical for tumor development. Responses to immune checkpoint inhibitors (ICIs) vary among patients, with a total response rate around 30%, and microbiome composition has been one of the factors linked to patient responsiveness to these treatments.
The intestinal microbiome directly influences host metabolism through secreting metabolites that aid in nutrient degradation, producing short-chain fatty acids, bile acids, amino acids, & vitamins, and generating various enzymes. With over 1,000 microbial species residing in the human gut, this community protects against pathogens, modulates immunity, and regulates metabolism, functioning almost like an endocrine organ. Functional metagenomics can reveal new microbial genes, pathways and host-microbiome interactions, helping us to understand what causes functional dysbiosis within the intestinal microbiome, and how to fix it.
Scientific breakthroughs in microbiome analysis have focused on understanding the interaction between the microbiome, immune system, and skin cells in different skin conditions, paving the way for future cosmetic products.
The skin hosts a diverse community of microorganisms known as the skin microbiota, which serve as a complex barrier against harmful external factors. This relationship involves constant communication between the microorganisms and the immune system, maintaining a healthy balance that is essential for skin health. Key bacterial groups on the skin include Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes. Specific bacteria, such as Staphylococcus epidermidis and Propionibacterium acnes, have been found to inhibit the growth of harmful bacteria and promote skin barrier function.
Changes in the microenvironment of the skin can disrupt the balance of skin microbial communities, reduce diversity, and turn otherwise useful bacteria into opportunistic pathogens, leading to skin issues like atopic dermatitis, psoriasis, rosacea, xerosis and acne. Research on cosmetics containing pre, pro, and postbiotics - usually microbiome derived compounds - is growing, showing promise in treating skin conditions. Modern dermocosmetics aim to not only repair but also reverse wear and tear of skin, by enhancing skin condition, repairing injured skin, encouraging wound healing, lessening uneven skin pigmentation, preventing skin aging, and maintaining a healthy equilibrium of skin defenses.
The future of skincare seems to be leading towards personalized microbiome-derived cosmetic solutions for individualized skincare routines. Spatial analysis, or 3D skin cartography can help create these personalized maps, to help us understand how different microbial populations are arranged, how they interact with each other and with skin cells. This involves mapping and visualizing the distribution of microorganisms and metabolites on the skin's surface in 3 dimensions with techniques such as spatial multiplexing - which uses immunohistochemistry to detect protein expression and cell distribution in a tissue sample. Better the control of background noise, image quality, resolution, & image processing/quantification algorithms, better the results.
Strand has developed a pipeline, equipped with advanced Cellpose3 technology, that improves cell segmentation and leads to better quantitative processing for the spatial multiplexing platform of a US-based spatial biology instrument company. Not only this, Strand offers solutions for multiple challenges in this process. For instance end-to-end pipelines to handle sequencing-based and image-based spatial transcriptomics; options for quality control such as specialized filters, automated statistical calculations; composite images generation from layered 2D images; batch effect correction; comparative analysis; and cell type annotation.
The vast potential of microbiome analysis can change the game for entire fields. From uncovering key biomarkers for disease, developing targeted therapies, and creating state of the art skincare, Strand can help transform microbiome data into actionable strategies for your venture.
Explore the rest of the blog series below: