Compliance and Validation Services
Compliance and Validation Services
Regulatory compliance is an integral part of safe, reliable, and sound research. Keeping up to with regulations can be a challenging process, scroll on to learn how Strand can help you easily stay up to date.
21CFR11
21CFR11
What is 21CFR11?
- 21 CFR (Code of Federal Regulations) Part 11 is the FDA rule that makes electronic records and signatures legally valid and secure.
- At Strand, we fully comply with 21CFR11, ensuring that all our electronic records and electronic signatures meet FDA requirements for security, integrity, and traceability.
- Our systems are validated, access-controlled, and designed to safeguard data accuracy and reliability.
- If your business handles regulated data, compliance is essential to avoid risks and ensure smooth FDA audits. We help you achieve 21 CFR Part 11 compliance with confidence.
What companies fall under the purview of 21CFR11?
Any FDA-regulated company like pharma, biotech, medical devices and CROs, that uses electronic records or signatures instead of paper to meet FDA requirements must comply with 21 CFR Part 11.
IQ. OQ. PQ
IQ. OQ. PQ
Installation
Qualification (IQ)
Verification that installation is carried out correctly. This can include:
- Physical verification of accessories, components & utilities
- Checking documentation, review manuals, tech specs, drawings etc
- Ensuring environmental requirements are met, such as temperature, humidity and ventilation
Operational
Qualification (OQ)
Testing to ensure equipment & systems function correctly and reliably under normal operating conditions. Some checks include:
- Testing that equipment systems function as intended
- Performance testing to ensure workload capacity is met
- Checking accuracy, precision & stability
- Environmental testing to check that the system can perform under different environmental conditions
Performance
Qualification (PQ)
Ensuring consistent performance against predefined requirements and specifications. This testing can include:
- Testing over a period of time to check for consistency and reproducibility of results
- Ensuring training and documentation systems, such as SOPs and maintenance manuals are created
- Validating the traceability and integrity of data
- Incorporating data backups, archiving & audit trails
“V” Model of Qualification and Validation
“V” Model of Qualification and Validation
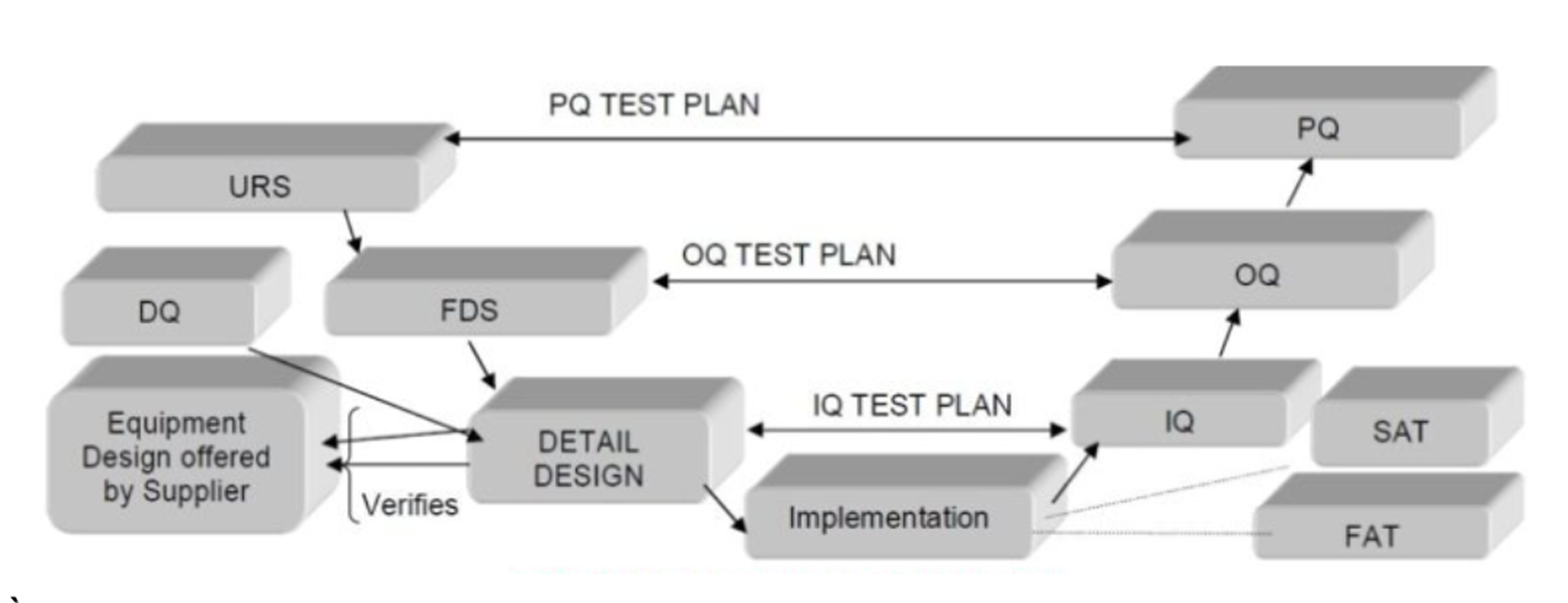
SOP – Standard Operating Procedure
A documented, detailed set of instructions to ensure processes are carried out consistently and in compliance with standards.
FAT – Factory Acceptance Test
A set of tests conducted at the vendor’s site to verify that equipment, systems, or instruments meet the agreed specifications before shipment.
SAT – Site Acceptance Test
Testing carried out at the client’s site after installation to ensure equipment or systems perform correctly in their actual operating environment.
DQ – Design Qualification
A documented verification that the proposed design of facilities, systems, or equipment is suitable for the intended purpose and meets regulatory requirements.
FDS – Functional Design Specification
A detailed description of how a system, instrument, or software should function, outlining requirements, inputs, outputs, and interactions.
URS – User Requirement Specification
A document defining what the end user expects from a system or equipment, typically serving as the basis for design and validation activities
Case Studies
Case Studies
We were recently approached to review and ensure that the HRD analysis component of a company’s Dragen TSO500 pipeline is correctly installed, operates as intended, and produces reliable results, following IQ, OQ and PQ standards, so that it could meet performance standards for precision in HRD scoring, as is summarised in the table below.
| Step | Installation Qualification (IQ) | Operational Qualification (OQ) | Performance Qualification (PQ) |
|---|---|---|---|
| Objective | Confirm that the HRD analysis component within the Dragen TSO500 pipeline is installed accurately and all prerequisite configurations are in place | Validate that the TSO500 HRD component operates correctly, generating expected outputs under standard conditions. | Demonstrate that the TSO500 HRD component consistently provides accurate, reliable, and reproducible HRD results. |
To learn more about the work we did, check out this case study!
Strand’s StrandAdvantage 500 test (SA500) is a Research Use Only (RUO) test panel procured from Illumina, USA, and validated as a Laboratory Developed Test (LDT) at Strand Life Sciences’s Clinical reference and R&D lab at Bangalore, India.
As part of its development, SA500 went through rigorous checks, reviewing both the wet lab and bioinformatics workflow before thorough validation of the results was carried out. To learn more about both SA500 and the validation, check out this validation report.
CASE STUDY 01
TSO500 - IQ OQ PQ Validation
We were recently approached to review and ensure that the HRD analysis component of a company’s Dragen TSO500 pipeline is correctly installed, operates as intended, and produces reliable results, following IQ, OQ and PQ standards, so that it could meet performance standards for precision in HRD scoring, as is summarised in the table below.
| Step | Installation Qualification (IQ) | Operational Qualification (OQ) | Performance Qualification (PQ) |
|---|---|---|---|
| Objective | Confirm that the HRD analysis component within the Dragen TSO500 pipeline is installed accurately and all prerequisite configurations are in place | Validate that the TSO500 HRD component operates correctly, generating expected outputs under standard conditions. | Demonstrate that the TSO500 HRD component consistently provides accurate, reliable, and reproducible HRD results. |
To learn more about the work we did, check out this case study!
CASE STUDY 02
SA500 Validation
Strand’s StrandAdvantage 500 test (SA500) is a Research Use Only (RUO) test panel procured from Illumina, USA, and validated as a Laboratory Developed Test (LDT) at Strand Life Sciences’s Clinical reference and R&D lab at Bangalore, India.
As part of its development, SA500 went through rigorous checks, reviewing both the wet lab and bioinformatics workflow before thorough validation of the results was carried out. To learn more about both SA500 and the validation, check out this validation report.



