Quantitative polymerase chain reaction (qPCR) is a well-established technique for high-throughput quantification of target DNA sequences. In infectious disease testing, qPCR is indispensable for rapid and accurate pathogen identification. Despite its widespread use and relevance for clinical decisions, automated solutions in the qPCR workflow are limited, causing delays and increased labor for laboratory scientists.
Strand's software team has recently developed an end-to-end qPCR software solution (PQRS) for infectious disease reporting.
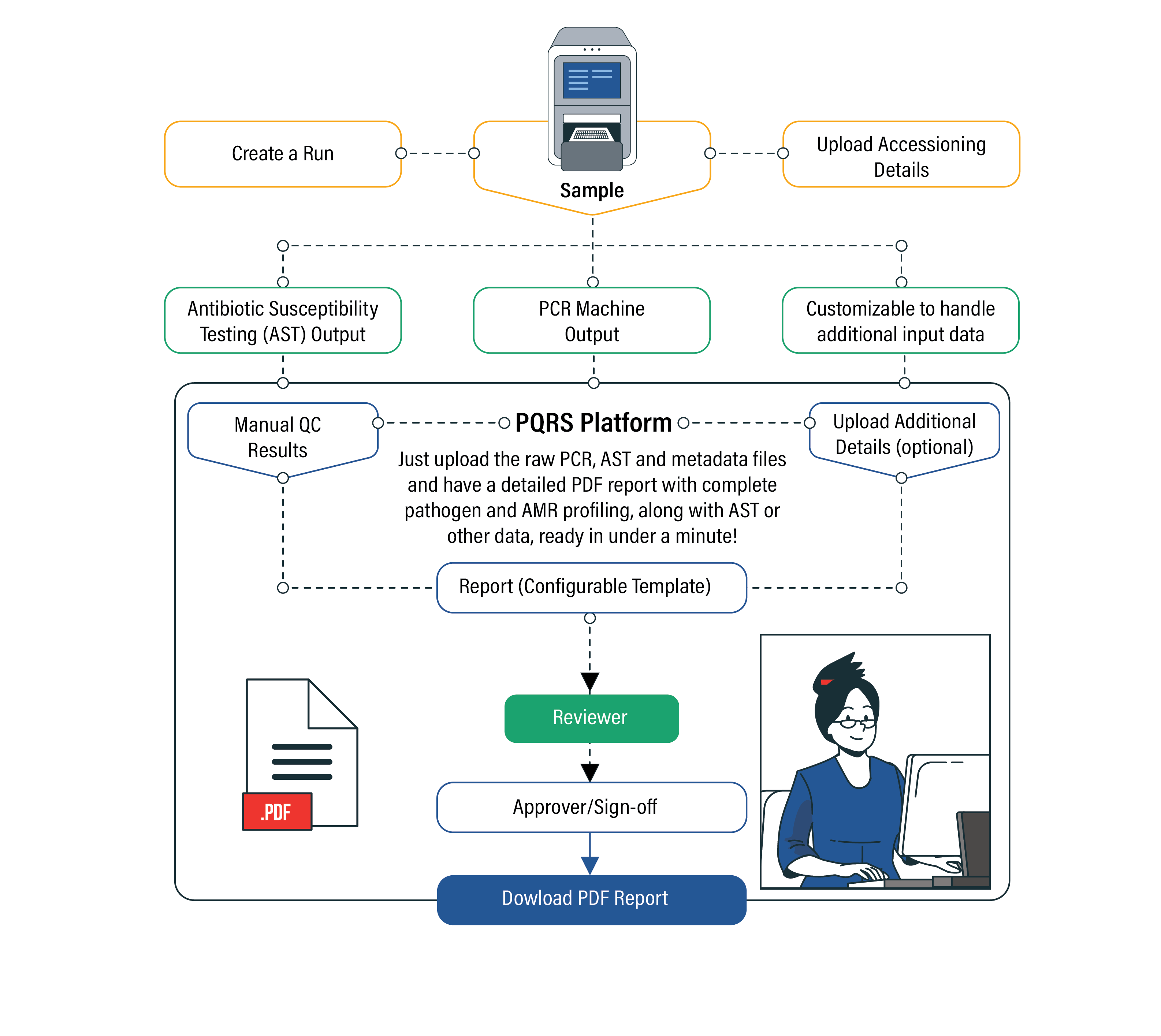
The process is initiated by the input of qPCR output files, which could be antibiotic susceptibility testing (AST) files, raw result files, or any other input data file.
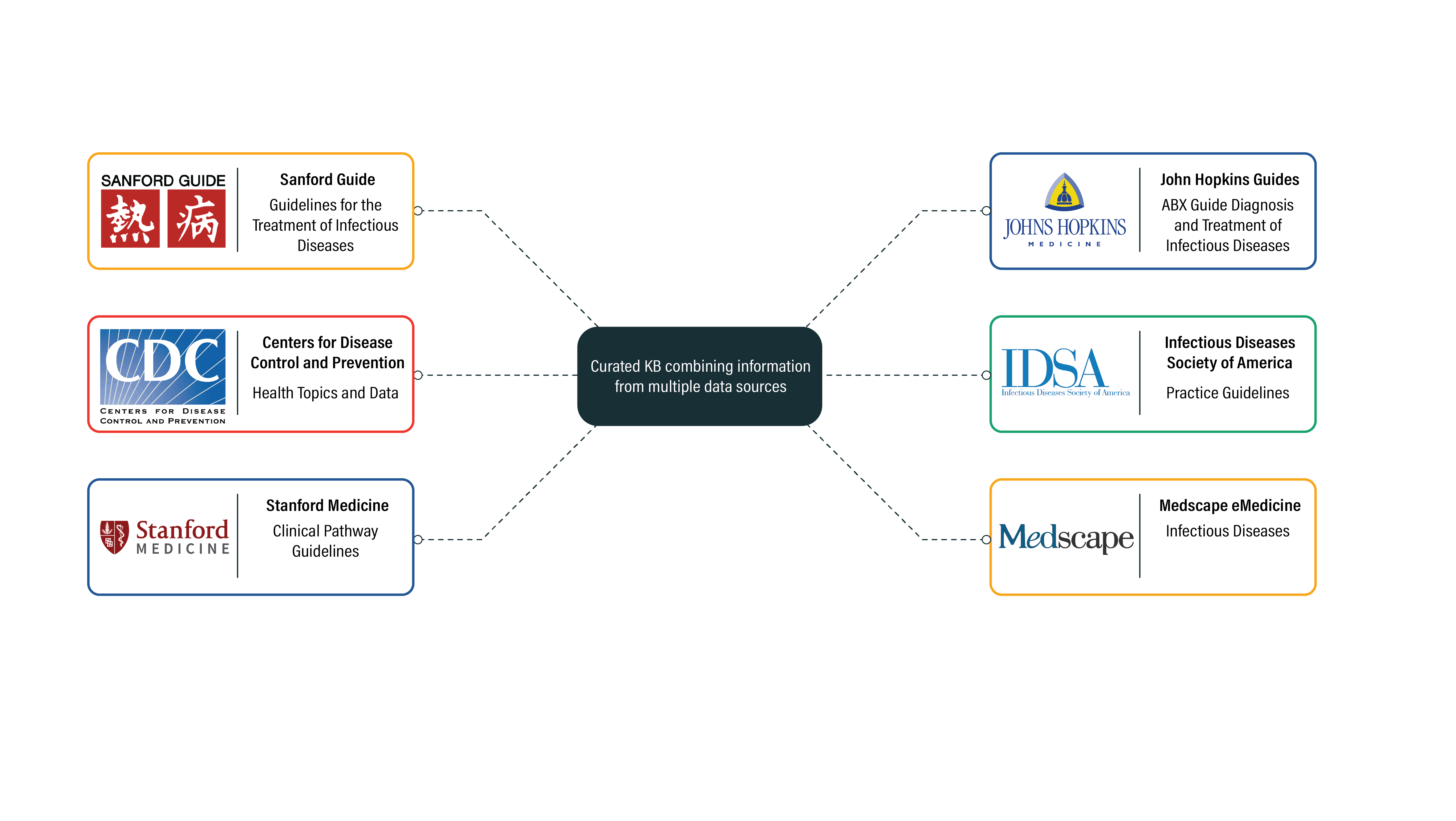
Next, a report based on a configurable template is compiled and generated. The platform leverages a curated knowledge base drawing from several key reference databases.
A quick review by a Senior Scientist follows, and the report is made available as a PDF download. It is worth highlighting that the turnaround time from input to report generation is just under a minute
To learn more about how the PQRS platform facilitates infectious disease reporting specifically, urinary tract infection (UTI) reporting, join us for an insightful discussion with Dr. Federica Gibellini and learn how AccessDx Lab's UTI panel and Strand's PQRS portal work together to enhance point-of-care UTI testing.

Catch the talk at PMWC - Precision Medicine World Conference in Silicon Valley, California, on Wednesday, January 24th, at 3:30 PM PST!